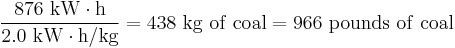Coal
| Sedimentary Rock | |
Anthracite coal |
|
| Composition | |
|---|---|
| Primary | carbon |
| Secondary | hydrogen, sulfur, oxygen, nitrogen |
Coal is a combustible black or brownish-black sedimentary rock usually occurring in rock strata in layers or veins called coal beds or coal seams. The harder forms, such as anthracite coal, can be regarded as metamorphic rock because of later exposure to elevated temperature and pressure. Coal is composed primarily of carbon along with variable quantities of other elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.[1]
Throughout history, coal has been a useful resource for human consumption. It is primarily burned as a fossil fuel for the production of electricity and/or heat, and is also used for industrial purposes such as refining metals. Coal forms when dead plant matter is converted into peat, which in turn is converted into lignite, then anthracite. This involves biological and geological processes that take place over a long period of time.
Top hard and brown coal producers in 2010 (2009) were (Mt): China 3,162 (2,971), United States 997 (985), India 571 (571), Australia 420 (399), Indonesia 336 (301), Russia 324 (297), South Africa 255 (247), Poland 134 (135), Kazakhstan 111 (101), and Colombia 74 (73).[2]
Contents |
Formation
About 300 million years ago, the earth had dense forests in low-lying wetland areas. Due to natural processes, like flooding, these forests got buried under the soil. As more and more soil deposited over them, they were compressed. The temperature also rose as they sank deeper and deeper. For the process to continue, the plant matter was protected from biodegradation and oxidization, usually by mud or acidic water. This traps the carbon in immense peat bogs that are eventually covered and deeply buried by sediments. Under high pressure and high temperature dead vegetation got slowly converted to coal. As coal contains mainly carbon, the conversion of dead vegetation into coal is called carbonization.[3]
The wide shallow seas of the Carboniferous period provided ideal conditions for coal formation, although coal is known from most geological periods. The exception is the coal gap in the Lower Triassic, where coal is rare: presumably a result of the mass extinction which prefaced this era. Coal is known from Precambrian strata, which predate land plants: this coal is presumed to have originated from algal residues.[4][5]
Coal, a fossil fuel, is the largest source of energy for the generation of electricity worldwide, as well as one of the largest worldwide anthropogenic sources of carbon dioxide releases. Gross carbon dioxide emissions from coal usage are slightly more than those from petroleum and about double the amount from natural gas.[6] Coal is extracted from the ground by mining, either underground by shaft mining through the seams or in open pits.
Types
As geological processes apply pressure to dead biotic material over time, under suitable conditions it is transformed successively into:
- Peat, considered to be a precursor of coal, has industrial importance as a fuel in some regions, for example, Ireland and Finland. In its dehydrated form, peat is a highly effective absorbent for fuel and oil spills on land and water. It is also used as a conditioner for soil to make it more able to retain and slow release water.
- Lignite or brown coal, is the lowest rank of coal and used almost exclusively as fuel for electric power generation. Jet is a compact form of lignite that is sometimes polished and has been used as an ornamental stone since the Upper Palaeolithic
- Sub-bituminous coal, whose properties range from those of lignite to those of bituminous coal, is used primarily as fuel for steam-electric power generation and is an important source of light aromatic hydrocarbons for the chemical synthesis industry.
- Bituminous coal is dense sedimentary rock, black but sometimes dark brown often with well-defined bands of bright and dull material, used primarily as fuel in steam-electric power generation, with substantial quantities used for heat and power applications in manufacturing and to make coke
- Steam coal is a grade between bituminous coal and anthracite, once widely used as a fuel for steam locomotives. In this specialized use it is sometimes known as sea-coal in the U.S.[7] Small steam coal (dry small steam nuts or DSSN) was used as a fuel for domestic water heating
- Anthracite, the highest rank of coal is a harder, glossy, black coal used primarily for residential and commercial space heating. It may be divided further into metamorphically altered bituminous coal and petrified oil, as from the deposits in Pennsylvania
- Graphite, technically the highest rank is difficult to ignite and is not commonly used as fuel: it is mostly used in pencils and, when powdered, as a lubricant.
The classification of coal is generally based on the content of volatiles. However, the exact classification varies between countries. According to the German classification, coal is classified as follows:[8]
| German Classification | English Designation | Volatiles % | C Carbon % | H Hydrogen % | O Oxygen % | S Sulfur % | Heat content kJ/kg |
|---|---|---|---|---|---|---|---|
| Braunkohle | Lignite | 45-65 | 60-75 | 6.0-5.8 | 34-17 | 0.5-3 | <28470 |
| Flammkohle | Flame coal | 40-45 | 75-82 | 6.0-5.8 | >9.8 | ~1 | <32870 |
| Gasflammkohle | Gas flame coal | 35-40 | 82-85 | 5.8-5.6 | 9.8-7.3 | ~1 | <33910 |
| Gaskohle | Gas coal | 28-35 | 85-87.5 | 5.6-5.0 | 7.3-4.5 | ~1 | <34960 |
| Fettkohle | Fat coal | 19-28 | 87.5-89.5 | 5.0-4.5 | 4.5-3.2 | ~1 | <35380 |
| Esskohle | Forge coal | 14-19 | 89.5-90.5 | 4.5-4.0 | 3.2-2.8 | ~1 | <35380 |
| Magerkohle | Non baking coal | 10-14 | 90.5-91.5 | 4.0-3.75 | 2.8-3.5 | ~1 | 35380 |
| Anthrazit | Anthracite | 7-12 | >91.5 | <3.75 | <2.5 | ~1 | <35300 |
| Percent by weight | |||||||
The middle six grades in the table represent a progressive transition from the English-language sub-bituminous to bituminous coal, while the last class is an approximate equivalent to anthracite, but more inclusive (the U.S. anthracite has < 6% volatiles).
Cannel coal (sometimes called "candle coal"), is a variety of fine-grained, high-rank coal with significant hydrogen content. It consists primarily of "exinite" macerals, now termed "liptinite".
Hilt's Law
Hilt's Law is a geological term that states that, in a small area, the deeper the coal, the deeper its rank (grade).[9] The law holds true if the thermal gradient is entirely vertical, but metamorphism may cause lateral changes of rank, irrespective of depth.
Early uses as fuel
The earliest reference to the use of coal as fuel is from the geological treatise On stones (Lap. 16) by the Greek scientist Theophrastus (c. 371–287 BC):
Outcrop coal was used in Britain during the Bronze Age (3000–2000 BC), where it has been detected as forming part of the composition of funeral pyres.[11][12] In Roman Britain, with the exception of two modern fields, "the Romans were exploiting coals in all the major coalfields in England and Wales by the end of the second century AD".[13] Evidence of trade in coal (dated to about AD 200) has been found at the inland port of Heronbridge, near Chester, and in the Fenlands of East Anglia, where coal from the Midlands was transported via the Car Dyke for use in drying grain.[14] Coal cinders have been found in the hearths of villas and military forts, particularly in Northumberland, dated to around AD 400. In the west of England contemporary writers described the wonder of a permanent brazier of coal on the altar of Minerva at Aquae Sulis (modern day Bath) although in fact easily accessible surface coal from what became the Somerset coalfield was in common use in quite lowly dwellings locally.[15] Evidence of coal's use for iron-working in the city during the Roman period has been found.[16] In Eschweiler, Rhineland, deposits of bituminous coal were used by the Romans for the smelting of iron ore.[13]
There is no evidence that the product was of great importance in Britain before the High Middle Ages, after about AD 1000.[17] Mineral coal came to be referred to as "seacoal" in the 13th century; the wharf where the material arrived in London was known as Seacoal Lane, so identified in a charter of King Henry III granted in 1253.[18] Initially the name was given because much coal was found on the shore, having fallen from the exposed coal seams on cliffs above or washed out of underwater coal outcrops,[17] but by the time of Henry VIII it was understood to derive from the way it was carried to London by sea.[19] In 1257–59, coal from Newcastle was shipped to London for the smiths and lime-burners building Westminster Abbey.[17] Seacoal Lane and Newcastle Lane where coal was unloaded at wharves along the River Fleet, are still in existence.[20] (See Industrial processes below for modern uses of the term.)
These easily accessible sources had largely become exhausted (or could not meet the growing demand) by the 13th century, when underground mining from shafts or adits was developed.[11] The alternative name was "pitcoal," because it came from mines. It was, however, the development of the Industrial Revolution that led to the large-scale use of coal, as the steam engine took over from the water wheel. In 1700, 5/6 of the world's coal was mined in Britain. Without coal, Britain would have run out of suitable sites for watermills by the 1830s.[21] In 1947, there were some 750,000 miners,[22] but by 2004 this had shrunk to some 5,000 miners working in around 20 collieries.[23]
In ancient China, coal was used as fuel by the 4th century AD, but there was little extensive use until the 11th century.[24]
Uses today
Coal as fuel
Coal is primarily used as a solid fuel to produce electricity and heat through combustion. World coal consumption was about 6.75 billion short tons in 2006[25] and is expected to increase 48% to 9.98 billion short tons by 2030.[26] China produced 2.38 billion tons in 2006. India produced about 447.3 million tons in 2006. 68.7% of China's electricity comes from coal. The USA consumes about 14% of the world total, using 90% of it for generation of electricity.[27]
When coal is used for electricity generation, it is usually pulverized and then combusted (burned) in a furnace with a boiler. The furnace heat converts boiler water to steam, which is then used to spin turbines which turn generators and create electricity. The thermodynamic efficiency of this process has been improved over time. Simple cycle steam turbines have topped out with some of the most advanced reaching about 35% thermodynamic efficiency for the entire process. Increasing the combustion temperature can boost this efficiency even further.[28] Old coal power plants, especially "grandfathered" plants, are significantly less efficient and produce higher levels of waste heat. At least 40% of the world's electricity comes from coal,[29] and in 2008 approximately 49% of the United States' electricity came from coal.[30] The emergence of the supercritical turbine concept envisions running a boiler at extremely high temperatures and pressures with projected efficiencies of 46%, with further theorized increases in temperature and pressure perhaps resulting in even higher efficiencies.[31]
An experimental way of coal combustion is in a form of coal-water slurry fuel (CWS, which was well-developed in Russia (since the Soviet Union time). CWS significantly reduces emissions saving the heating value of coal. Other ways to use coal are combined heat and power cogeneration and an MHD topping cycle.
The total known deposits recoverable by current technologies, including highly polluting, low energy content types of coal (i.e., lignite, bituminous), is sufficient for many years. However, consumption is increasing and maximal production could be reached within decades (see World Coal Reserves, below).
Coking coal and use of coke
Coke is a solid carbonaceous residue derived from low-ash, low-sulfur bituminous coal from which the volatile constituents are driven off by baking in an oven without oxygen at temperatures as high as 1,000 °C (1,832 °F) so that the fixed carbon and residual ash are fused together. Metallurgical coke is used as a fuel and as a reducing agent in smelting iron ore in a blast furnace.[32] The coking coal should be low in sulphur and phosphorus so that they do not migrate to the metal. The product is cast iron and is too rich in dissolved carbon, and so must be treated further to make steel.
The coke must be strong enough to resist the weight of overburden in the blast furnace, which is why coking coal is so important in making steel using the conventional route. However, the alternative route to is direct reduced iron, where any carbonaceous fuel can be used to make sponge or pelletised iron. Coke from coal is grey, hard, and porous and has a heating value of 24.8 million Btu/ton (29.6 MJ/kg). Some cokemaking processes produce valuable by-products that include coal tar, ammonia, light oils, and "coal gas".
Petroleum coke is the solid residue obtained in oil refining, which resembles coke but contains too many impurities to be useful in metallurgical applications.
Gasification
Coal gasification can be used to produce syngas, a mixture of carbon monoxide (CO) and hydrogen (H2) gas. This syngas can then be converted into transportation fuels like gasoline and diesel through the Fischer-Tropsch process. This technology is currently used by the Sasol chemical company of South Africa to make gasoline from coal and natural gas. Alternatively, the hydrogen obtained from gasification can be used for various purposes such as powering a hydrogen economy, making ammonia, or upgrading fossil fuels.
During gasification, the coal is mixed with oxygen and steam (water vapor) while also being heated and pressurized. During the reaction, oxygen and water molecules oxidize the coal into carbon monoxide (CO) while also releasing hydrogen (H2) gas. This process has been conducted in both underground coal mines and in coal refineries.
- (Coal) + O2 + H2O → H2 + CO
If the refiner wants to produce gasoline, the syngas is collected at this state and routed into a Fischer-Tropsch reaction. If hydrogen is the desired end-product, however, the syngas is fed into the water gas shift reaction where more hydrogen is liberated.
- CO + H2O → CO2 + H2
High prices of oil and natural gas are leading to increased interest in "BTU Conversion" technologies such as gasification, methanation and liquefaction. The Synthetic Fuels Corporation was a U.S. government-funded corporation established in 1980 to create a market for alternatives to imported fossil fuels (such as coal gasification). The corporation was discontinued in 1985.
In the past, coal was converted to make coal gas, which was piped to customers to burn for illumination, heating, and cooking. At present, the safer natural gas is used instead.
Liquefaction
Coal can also be converted into liquid fuels such as gasoline or diesel by several different processes. In the direct liquefaction processes, the coal is either hydrogenated or carbonized. Hydrogenation processes are the Bergius process,[33] the SRC-I and SRC-II (Solvent Refined Coal) processes and the NUS Corporation hydrogenation process.[34][35] In the process of low-temperature carbonization, coal is coked at temperatures between 360 °C (680 °F) and 750 °C (1,380 °F). These temperatures optimize the production of coal tars richer in lighter hydrocarbons than normal coal tar. The coal tar is then further processed into fuels. Alternatively, coal can be converted into a gas first, and then into a liquid, by using the Fischer-Tropsch process. An overview of coal liquefaction and its future potential is available.[36]
Coal liquefaction methods involve carbon dioxide (CO2) emissions in the conversion process. If coal liquefaction is done without employing either carbon capture and storage technologies or biomass blending, the result is lifecycle greenhouse gas footprints that are generally greater than those released in the extraction and refinement of liquid fuel production from crude oil. If CCS technologies are employed, reductions of 5-12% can be achieved in CTL plants and up to a 75% reduction is achievable when co-gasifying coal with commercially demonstrated levels of biomass (30% biomass by weight) in CBTL plants.[37] For most future synthetic fuel projects, Carbon dioxide sequestration is proposed to avoid releasing it into the atmosphere. Sequestration will, however, add to the cost of production. Currently all US and at least one Chinese synthetic fuel projects,[38] include sequestration in their process designs.
Refined coal
Refined coal is the product of a coal-upgrading technology that removes moisture and certain pollutants from lower-rank coals such as sub-bituminous and lignite (brown) coals. It is one form of several pre-combustion treatments and processes for coal that alter coal's characteristics before it is burned. The goals of pre-combustion coal technologies are to increase efficiency and reduce emissions when the coal is burned. Depending on the situation, pre-combustion technology can be used in place of or as a supplement to post-combustion technologies to control emissions from coal-fueled boilers.
Industrial processes
Finely ground bituminous coal, known in this application as sea coal, is a constituent of foundry sand. While the molten metal is in the mould the coal burns slowly, releasing reducing gases at pressure and so preventing the metal from penetrating the pores of the sand. It is also contained in mould wash, a paste or liquid with the same function applied to the mould before casting.[39] Sea coal can be mixed with the clay lining (the "bod") used for the bottom of a cupola furnace. When heated the coal decomposes and the bod becomes slightly friable, easing the process of breaking open holes for tapping the molten metal.[40]
Cultural usage
Coal is the official state mineral of Kentucky[41] (even though coal is not a mineral) and the official state rock of Utah.[42] Both U.S. states have a historic link to coal mining.
Some cultures uphold that children who misbehave will receive only a lump of coal from Santa Claus for Christmas in their stockings instead of presents.
It is also customary and lucky in Scotland and the North of England to give coal as a gift on New Year's Day. It happens as part of First-Footing and represents warmth for the year to come.
Coal as a traded commodity
In North America, Central Appalachian coal futures contracts are currently traded on the New York Mercantile Exchange (trading symbol QL). The trading unit is 1,550 short tons (1,410 t) per contract, and is quoted in U.S. dollars and cents per ton. Since coal is the principal fuel for generating electricity in the United States, coal futures contracts provide coal producers and the electric power industry an important tool for hedging and risk management.[43]
In addition to the NYMEX contract, the IntercontinentalExchange (ICE) has European (Rotterdam) and South African (Richards Bay) coal futures available for trading. The trading unit for these contracts is 5,000 tonnes (5,500 short tons), and are also quoted in U.S. dollars and cents per ton.[44]
The price of coal increased from around $30.00 per short ton in 2000 to around $150.00 per short ton as of September 2008. As of October 2008, the price per short ton had declined to $111.50. Prices further declined to $71.25 as of October 2010.[45]
Environmental effects
There are a number of adverse health[46] and environmental effects of coal burning[47] especially in power stations, and of coal mining. These effects include:
- Coal-fired power plants shortened nearly 24,000 lives a year in the United States, including 2,800 from lung cancer[48]
- Generation of hundreds of millions of tons of waste products, including fly ash, bottom ash, flue-gas desulfurization sludge, that contain mercury, uranium, thorium, arsenic, and other heavy metals
- Acid rain from high sulfur coal
- Interference with groundwater and water table levels
- Contamination of land and waterways and destruction of homes from fly ash spills such as Kingston Fossil Plant coal fly ash slurry spill
- Impact of water use on flows of rivers and consequential impact on other land-uses
- Dust nuisance
- Subsidence above tunnels, sometimes damaging infrastructure
- Uncontrollable underground fires which may burn for decades or centuries.
- Coal-fired power plants without effective fly ash capture are one of the largest sources of human-caused background radiation exposure
- Coal-fired power plants emit mercury, selenium, and arsenic which are harmful to human health and the environment[49]
- Release of carbon dioxide, a greenhouse gas, which causes climate change and global warming according to the IPCC and the EPA. Coal is the largest contributor to the human-made increase of CO2 in the air[50]
Economic aspects
Coal liquification is one of the backstop technologies that could potentially limit escalation of oil prices and mitigate the effects of transportation energy shortage that will occur under peak oil. This is contingent on liquefaction production capacity becoming large enough to satiate the very large and growing demand for petroleum. Estimates of the cost of producing liquid fuels from coal suggest that domestic U.S. production of fuel from coal becomes cost-competitive with oil priced at around $35 per barrel,[51] with the $35 being the break-even cost. With oil prices as low as around $40 per barrel in the U.S. as of December 2008, liquid coal lost some of its economic allure in the U.S., but will probably be re-vitalized, similar to oil sand projects, with an oil price around $70 per barrel.
United States
Some of the simplest economic costs of coal come in the form of subsidies and tax breaks which are not reflected in the market price of coal (for example the estimated $4.6 billion in coal-related subsidies in the 2009 stimulus package). Coal mining and combustion projects require major investments, and the risks and costs of those investments are often passed on to taxpayers via infrastructure subsidies and loan guarantees. An extreme example of this is the Healy Clean Coal Project (HCCP), which has cost the State of Alaska and the Federal Government nearly $300 million since the mid 1990s to evaluate experimental clean coal technology. Similarly, a recent study in Kentucky determined that the government spends $115 million more on subsidies for the coal industry in the state than it receives in taxes or other benefits.
Taxpayers also pay the costs of cleaning up environmental disasters caused by the coal industry. Cleanup of the recent coal ash spill in Tennessee is estimated to cost up to $1 billion, not including pending litigation. Now that the cleanup at this site has been taken over by the EPA under the Superfund law, most of this cost will be borne by the US taxpayer.
The health impacts of coal pollution have enormous economic costs, through health care costs and lost productivity. The Ontario government study estimated these costs as billions of dollars within Ontario alone. A similar recent study in West Virginia found that the cost associated with premature death due to coal mining was five times greater than all measurable economic benefits from the mining.
Other industries depend on the ecosystems coal mining destroys. This economic impact on industries such as recreational fishing, commercial fishing, and tourism is particularly relevant in Alaska. Almost 55,000 direct jobs (full time equivalent basis, FTE) are closely linked to the health of Alaska's ecosystems. These jobs make up more than a quarter of Alaskan FTE employment and produce almost $2.6 billion of income for Alaska workers. These 55,000 ecosystem-dependent jobs dwarf the 350 estimated jobs that would be created by a project such as the Chuitna Coal strip mine.
Negative effects on the economy lead to worse health in the population, which has an impact on health care costs, compounding the economic impact. Some people have used this to argue that coal has additional benefits to society. The argument is that coal provides cheap electricity, which is a boon to the economy, therefore health is improved, and health care costs are lowered. While this additional health effect should indeed be considered, it should be applied after the economic impacts discussed above. Once the costs of pollution, global warming, and habitat destruction are added to the benefits of cheap electricity, the economic impact of coal is no longer positive, and this additional health effect only makes it even more costly.
China
In China, due to an increasing need for liquid energy in the transportation sector, coal liquefaction projects were given high priority even during periods of oil prices below $40 per barrel.[52] This is probably because China prefers not to be dependent on foreign oil, instead utilizing its enormous domestic coal reserves. As oil prices were increasing during the first half of 2009, the coal liquefaction projects in China were again boosted, and these projects are profitable with an oil barrel price of $40.[53]
China is by far the largest producer of coal in the world.[54] It has now become the world's largest energy consumer[55] but relies on coal to supply about 70% of its energy needs.[56] An estimated 5 million people work in China's coal-mining industry.[57]
Among commercially mature technologies, advantages for indirect coal liquefaction over direct coal liquefaction are reported by Williams and Larson (2003).
Energy density
The energy density of coal, i.e. its heating value, is roughly 24 megajoules per kilogram.[58]
The energy density of coal can also be expressed in kilowatt-hours, the units that electricity is most commonly sold in, per units of mass to estimate how much coal is required to power electrical appliances. One kilowatt-hour is 3.6 MJ, so the energy density of coal is 6.67 kW·h/kg. The typical thermodynamic efficiency of coal power plants is about 30%, so of the 6.67 kW·h of energy per kilogram of coal, 30% of that—2.0 kW·h/kg—can successfully be turned into electricity; the rest is waste heat. So coal power plants obtain approximately 2.0 kW·h per kilogram of burned coal.
As an example, running one 100-watt lightbulb for one year requires 876 kW·h (100 W × 24 h/day × 365 day/year = 876000 W·h = 876 kW·h). Converting this power usage into physical coal consumption:
For a coal power plant with a 40% efficiency, it takes 325 kg (714 lb) of coal to power a 100 W lightbulb for one year.[59] One should also take into account transmission and distribution losses caused by resistance and heating in the power lines, which is in the order of 5–10%, depending on distance from the power station and other factors.
Carbon intensity
Commercial coal has a carbon content of at least 70%. Coal with a heating value of 6.67 kWh per kilogram as quoted above has a carbon content of roughly 80%, which is
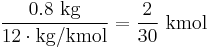 , where 1 mol equals to NA (Avogadro Number) atoms.
, where 1 mol equals to NA (Avogadro Number) atoms.
Carbon combines with oxygen in the atmosphere during combustion, producing carbon dioxide, with an atomic weight of (12 + 16 × 2 = 44 kg/kmol). The CO2 released to air for each kilogram of incinerated coal is therefore
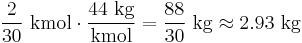 .
.
This can be used to calculate an emission factor for CO2 from the use of coal power. Since the useful energy output of coal is about 31% of the 6.67 kWh/kg(coal),[60] the burning of 1 kg of coal produces about 2 kWh of electrical energy. Since 1 kg coal emits 2.93 kg CO2, the direct CO2 emissions from coal power are 1.47 kg/kWh, or about 0.407 kg/MJ.
The U.S. Energy Information Agency's 1999 report on CO2 emissions for energy generation,[61] quotes a lower emission factor of 0.963 kg CO2/kWh for coal power. The same source gives a factor for oil power in the U.S. of 0.881 kg CO2/kWh, while natural gas has 0.569 kg CO2/kWh. Estimates for specific emission from nuclear power, hydro, and wind energy vary, but are about 100 times lower.
Underground fires
There are thousands of coal fires burning around the world.[62] Those burning underground can be difficult to locate and many cannot be extinguished. Fires can cause the ground above to subside, their combustion gases are dangerous to life, and breaking out to the surface can initiate surface wildfires. Coal seams can be set on fire by spontaneous combustion or contact with a mine fire or surface fire. Lightning strikes are an important source of ignition, the coal continues to burn slowly back into the seam until oxygen (air) can no longer reach the flame front. A grass fire in a coal area can set dozens of coal seams on fire.[63][64] Coal fires in China burn an estimated 120 million tons of coal a year, emitting 360 million metric tons of CO2, amounting to 2-3% of the annual worldwide production of CO2 from fossil fuels.[65][66] In Centralia, Pennsylvania (a borough located in the Coal Region of the United States), an exposed vein of coal ignited in 1962 due to a trash fire in the borough landfill, located in an abandoned anthracite strip mine pit. Attempts to extinguish the fire were unsuccessful, and it continues to burn underground to this day. The Australian Burning Mountain was originally believed to be a volcano, but the smoke and ash comes from a coal fire that has been burning for some 6,000 years.[67]
At Kuh i Malik in Yagnob Valley, Tajikistan, coal deposits have been burning for thousands of years, creating vast underground labyrinths full of unique minerals, some of them very beautiful. Local people once used this method to mine ammoniac. This place has been well-known since the time of Herodotus, but European geographers misinterpreted the Ancient Greek descriptions as the evidence of active volcanism in Turkestan (up to the 19th century, when the Russian army invaded the area).
The reddish siltstone rock that caps many ridges and buttes in the Powder River Basin (Wyoming), and in western North Dakota is called porcelanite, which also may resemble the coal burning waste "clinker" or volcanic "scoria".[68] Clinker is rock that has been fused by the natural burning of coal. In the Powder River Basin approximately 27 to 54 billion tons of coal burned within the past three million years.[69] Wild coal fires in the area were reported by the Lewis and Clark Expedition as well as explorers and settlers in the area.[70]
Production trends
In 2006, China was the top producer of coal with 38% share followed by the USA and India, according to the British Geological Survey.
World coal reserves
The 930 billion short tons of recoverable coal reserves estimated by the Energy Information Administration are equal to about 4,116 BBOE (billion barrels of oil equivalent). The amount of coal burned during 2007 was estimated at 7.075 billion short tons, or 133.179 quadrillion BTU's.[71] This is an average of 18.8 million BTU per short ton. In terms of heat content, this is about 57,000,000 barrels (9,100,000 m3) of oil equivalent per day. By comparison in 2007, natural gas provided 51,000,000 barrels (8,100,000 m3) of oil equivalent per day, while oil provided 85,800,000 barrels (13,640,000 m3) per day.
BP, in its 2007 report, estimated at 2006 end that there were several billion tons of proven coal reserves worldwide, or 147 years reserves-to-production ratio. This figure only includes reserves classified as "proven"; exploration drilling programs by mining companies, particularly in under-explored areas, are continually providing new reserves. In many cases, companies are aware of coal deposits that have not been sufficiently drilled to qualify as "proven". However, some nations haven't updated their information and assume reserves remain at the same levels even with withdrawals. Speculative projections predict that global peak coal production may occur sometime around 2025 at 30 percent above current production, depending on future coal production rates.[72]
Of the three fossil fuels, coal has the most widely distributed reserves; coal is mined in over 100 countries, and on all continents except Antarctica. The largest reserves are found in the USA, Russia, China, India and Australia. Note the table below.
| Country | Bituminous & Anthracite | SubBituminous | Lignite | TOTAL | Percentage of World Total |
|---|---|---|---|---|---|
| United States | 108,501 | 98,618 | 30,176 | 237,295 | 22.6 |
| Russia | 49,088 | 97,472 | 10,450 | 157,010 | 14.4 |
| China | 62,200 | 33,700 | 18,600 | 114,500 | 12.6 |
| Australia | 37,100 | 2,100 | 37,200 | 76,500 | 8.9 |
| India | 56,100 | 0 | 4,500 | 60,600 | 7.0 |
| Germany | 99 | 0 | 40,600 | 40,699 | 4.7 |
| Ukraine | 15,351 | 16,577 | 1,945 | 33,873 | 3.9 |
| Kazakhstan | 21,500 | 0 | 12,100 | 33,600 | 3.9 |
| South Africa | 30,156 | 0 | 0 | 30,156 | 3.5 |
| Serbia | 9 | 361 | 13,400 | 13,770 | 1.6 |
| Colombia | 6,366 | 380 | 0 | 6,746 | 0.8 |
| Canada | 3,474 | 872 | 2,236 | 6,528 | 0.8 |
| Poland | 4,338 | 0 | 1,371 | 5,709 | 0.7 |
| Indonesia | 1,520 | 2,904 | 1,105 | 5,529 | 0.6 |
| Brazil | 0 | 4,559 | 0 | 4,559 | 0.5 |
| Greece | 0 | 0 | 3,020 | 3,020 | 0.4 |
| Bosnia and Herzegovina | 484 | 0 | 2,369 | 2,853 | 0.3 |
| Mongolia | 1,170 | 0 | 1,350 | 2,520 | 0.3 |
| Bulgaria | 2 | 190 | 2,174 | 2,366 | 0.3 |
| Pakistan | 0 | 166 | 1,904 | 2,070 | 0.3 |
| Turkey | 529 | 0 | 1,814 | 2,343 | 0.3 |
| Uzbekistan | 47 | 0 | 1,853 | 1,900 | 0.2 |
| Hungary | 13 | 439 | 1,208 | 1,660 | 0.2 |
| Thailand | 0 | 0 | 1,239 | 1,239 | 0.1 |
| Mexico | 860 | 300 | 51 | 1,211 | 0.1 |
| Iran | 1,203 | 0 | 0 | 1,203 | 0.1 |
| Czech Republic | 192 | 0 | 908 | 1,100 | 0.1 |
| Kyrgyzstan | 0 | 0 | 812 | 812 | 0.1 |
| Albania | 0 | 0 | 794 | 794 | 0.1 |
| North Korea | 300 | 300 | 0 | 600 | 0.1 |
| New Zealand | 33 | 205 | 333-7,000 | 571-15,000[74] | 0.1 |
| Spain | 200 | 300 | 30 | 530 | 0.1 |
| Laos | 4 | 0 | 499 | 503 | 0.1 |
| Zimbabwe | 502 | 0 | 0 | 502 | 0.1 |
| Argentina | 0 | 0 | 500 | 500 | 0.1 |
| All others | 3,421 | 1,346 | 846 | 5,613 | 0.7 |
| Total world | 404,762 | 260,789 | 195,387 | 860,938 | 100 |
Major coal producers
The reserve life is an estimate based only on current production levels and proved reserves level for the countries shown, and makes no assumptions of future production or even current production trends. Countries with annual production higher than 100 million tonnes are shown. For comparison, data for the European Union is also shown. Shares are based on data expressed in tonnes oil equivalent.
| Country | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | Share | Reserve Life (years) |
|---|---|---|---|---|---|---|---|---|---|---|
| China | 1834.9 | 2122.6 | 2349.5 | 2528.6 | 2691.6 | 2802.0 | 2973.0 | 3240.0 | 48.3 % | 35 |
| USA | 972.3 | 1008.9 | 1026.5 | 1054.8 | 1040.2 | 1063.0 | 975.2 | 984.6 | 14.8 % | 241 |
| India | 375.4 | 407.7 | 428.4 | 449.2 | 478.4 | 515.9 | 556.0 | 569.9 | 5.8 % | 106 |
| EU | 637.2 | 627.6 | 607.4 | 595.1 | 592.3 | 563.6 | 538.4 | 535.7 | 4.2 % | 105 |
| Australia | 350.4 | 364.3 | 375.4 | 382.2 | 392.7 | 399.2 | 413.2 | 423.9 | 6.3 % | 180 |
| Russia | 276.7 | 281.7 | 298.3 | 309.9 | 313.5 | 328.6 | 301.3 | 316.9 | 4.7 % | 495 |
| Indonesia | 114.3 | 132.4 | 152.7 | 193.8 | 216.9 | 240.2 | 256.2 | 305.9 | 5.0 % | 18 |
| South Africa | 237.9 | 243.4 | 244.4 | 244.8 | 247.7 | 252.6 | 250.6 | 253.8 | 3.8 % | 119 |
| Germany | 204.9 | 207.8 | 202.8 | 197.1 | 201.9 | 192.4 | 183.7 | 182.3 | 1.2 % | 223 |
| Poland | 163.8 | 162.4 | 159.5 | 156.1 | 145.9 | 144.0 | 135.2 | 133.2 | 1.5 % | 43 |
| Kazakhstan | 84.9 | 86.9 | 86.6 | 96.2 | 97.8 | 111.1 | 100.9 | 110.8 | 1.5 % | 303 |
| Total World | 5,301.3 | 5,716.0 | 6,035.3 | 6,342.0 | 6,573.3 | 6,795.0 | 6,880.8 | 7,273.3 | 100 % | 118 |
Major coal exporters
Countries with annual export higher than 10 million tonnes are shown.
| Country | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | Share |
|---|---|---|---|---|---|---|---|---|
| Australia | 238.1 | 247.6 | 255.0 | 255.0 | 268.5 | 278.0 | 288.5 | 26.5% |
| Indonesia | 107.8 | 131.4 | 142.0 | 192.2 | 221.9 | 228.2 | 261.4 | 24.0% |
| Russia | 41.0 | 55.7 | 98.6 | 103.4 | 112.2 | 115.4 | 130.9 | 12.0% |
| Colombia | 50.4 | 56.4 | 59.2 | 68.3 | 74.5 | 74.7 | 75.7 | 6.9% |
| South Africa | 78.7 | 74.9 | 78.8 | 75.8 | 72.6 | 68.2 | 73.8 | 6.8% |
| USA | 43.0 | 48.0 | 51.7 | 51.2 | 60.6 | 83.5 | 60.4 | 5.5% |
| China | 103.4 | 95.5 | 93.1 | 85.6 | 75.4 | 68.8 | 38.4 | 3.5% |
| Canada | 27.7 | 28.8 | 31.2 | 31.2 | 33.4 | 36.5 | 31.9 | 2.9% |
| Vietnam | 6.9 | 11.7 | 19.8 | 23.5 | 35.1 | 21.3 | 28.2 | 2.6% |
| Kazakhstan | 30.3 | 27.4 | 28.3 | 30.5 | 32.8 | 47.6 | 25.7 | 2.4% |
| Poland | 28.0 | 27.5 | 26.5 | 25.4 | 20.1 | 16.1 | 14.6 | 1.3% |
| Total | 713.9 | 764.0 | 936.0 | 1,000.6 | 1,073.4 | 1,087.3 | 1,090.8 | 100% |
Major coal importers
Countries with annual import higher than 30 million tonnes are shown.
| Country | 2006 | 2007 | 2008 | 2009 | Share |
|---|---|---|---|---|---|
| Japan | 199.7 | 209.0 | 206.0 | 182.1 | 17.5% |
| China | 42.0 | 56.2 | 44.5 | 151.9 | 14.5% |
| South Korea | 84.1 | 94.1 | 107.1 | 109.9 | 10.6% |
| India | 52.7 | 29.6 | 70.9 | 76.7 | 7.4% |
| Taiwan | 69.1 | 72.5 | 70.9 | 64.6 | 6.2% |
| Germany | 50.6 | 56.2 | 55.7 | 45.9 | 4.4% |
| United Kingdom | 56.8 | 48.9 | 49.2 | 42.2 | 4.1% |
| Total | 991.8 | 1,056.5 | 1,063.2 | 1,039.8 | 100% |
|
||||||||||||||||||||||||||
See also
- Asphaltene
- Biochar
- Biomass-coal
- Carbochemistry
- Coal assay
- Coal dust
- Coal Measure (stratigraphic unit)
- Coal mining
- Coal phase out
- Coal-tar
- Coalbed methane
- Fluidized bed combustion
- Major coal producing regions
- Mountaintop removal mining
- The Coal Question
- World Coal Association
References
- ^ Blander, M. "CALCULATIONS OF THE INFLUENCE OF ADDITIVES ON COAL COMBUSTION DEPOSITS". Argonne National Laboratory. p. 43. http://www.anl.gov/PCS/acsfuel/preprint%20archive/Files/Volumes/Vol34-2.pdf. Retrieved 17 December 2011.
- ^ IEA Key energy statistics 2010 [IEA Key World Energy Statistics 2011 pages 11, 21
- ^ Taylor, Thomas N; Taylor, Edith L; Krings, Michael (2009). Paleobotany: The biology and evolution of fossil plants. ISBN 9780123739728. http://books.google.ca/books?id=_29tNNeQKeMC&pg=PA18.
- ^ Tyler, S. A.; Barghoorn, E. S.; Barrett, L. P. (1957). "Anthracitic Coal from Precambrian Upper Huronian Black Shale of the Iron River District, Northern Michigan". Geological Society of America Bulletin 68 (10): 1293. doi:10.1130/0016-7606(1957)68[1293:ACFPUH]2.0.CO;2. ISSN 0016-7606.
- ^ Mancuso, J. J.; Seavoy, R. E. (1981). "Precambrian coal or anthraxolite; a source for graphite in high-grade schists and gneisses". Economic Geology 76 (4): 951–954. doi:10.2113/gsecongeo.76.4.951.
- ^ The EIA reports the following emissions in million metric tons of carbon dioxide:
- Nat gas: 5,840
- Petroleum: 10,995
- Coal: 11,357
- ^ Funk and Wagnalls, quoted in "sea-coal". Oxford English Dictionary (2 ed.). Oxford University Press. 1989.
- ^ Eberhard Lindner; Chemie für Ingenieure; Lindner Verlag Karlsruhe, S. 258
- ^ Hilt's Law according to answers.com]
- ^ Mattusch, Carol (2008): "Metalworking and Tools", in: Oleson, John Peter (ed.): The Oxford Handbook of Engineering and Technology in the Classical World, Oxford University Press, ISBN 978-0-19-518731-1, pp. 418–38 (432)
- ^ a b Britannica 2004: Coal mining: ancient use of outcropping coal
- ^ Needham, Joseph; Golas, Peter J (1999). Science and Civilisation in China. Cambridge University Press. pp. 186–91. ISBN 9780521580007.
- ^ a b Smith, A. H. V. (1997): "Provenance of Coals from Roman Sites in England and Wales", Britannia, Vol. 28, pp. 297–324 (322–4)
- ^ Salway, Peter (2001). A History of Roman Britain. Oxford University Press.
- ^ Forbes, RJ (1966): Studies in Ancient Technology. Brill Academic Publishers, Boston.
- ^ Cunliffe, Barry W. (1984). Roman Bath Discovered. London: Routledge. pp. 14–5, 194. ISBN 0710201966.
- ^ a b c Cantril, T. C. (1914). Coal Mining. Cambridge, England: Cambridge University Press. pp. 3–10. OCLC 156716838.
- ^ "coal, 5a". Oxford English Dictionary. Oxford University Press. 1 December 2010.
- ^ John Caius, quoted in Cantril (1914).
- ^ Trench, Richard; Hillman, Ellis (1993). London under London: a subterranean guide (Second ed.). London: John Murray. p. 33. ISBN 0-7195-5288-5.
- ^ Wrigley, EA. Continuity, Chance and Change.
- ^ "The fall of King Coal". BBC News. December 6, 1999. http://news.bbc.co.uk/2/hi/business/551544.stm.
- ^ "The miners' strike revisited". Inside Out (BBC). http://www.bbc.co.uk/insideout/eastmidlands/series5/miners_strike_coal.shtml.
- ^ Read, Thomas T (1939–40). "The Earliest Industrial Use of Coal". Transactions of the Newcomen Society 20: p. 119.
- ^ World coal consupmption 1980-2006 October 2008 EIA statistics
- ^ EIA, World Energy Projections Plus (2009)
- ^ http://www.eia.doe.gov/cneaf/coal/page/special/feature.html
- ^ "Fossil Power Generation". Siemens AG. http://w1.siemens.com/responsibility/en/environment/portfolio/fossil_power_generation.htm. Retrieved 2009-04-23.
- ^ http://www.worldcoal.org/pages/content/index.asp?PageID=188
- ^ "Figure ES 1. U.S. Electric Power Industry Net Generation". Electric Power Annual with data for 2008. U.S. Energy Information Administration. 2010-01-21. http://www.eia.doe.gov/cneaf/electricity/epa/figes1.html. Retrieved 2010-11-07.
- ^ "Balancing economics and environmental friendliness - the challenge for supercritical coal-fired power plants with highest steam parameters in the future" (PDF). http://www.powergeneration.siemens.com/download/pool/PGE2005_BalancingEconomics.pdf. Retrieved 2006-10-23.
- ^ Blast furnace steelmaking cost model
- ^ Robert Haul: Friedrich Bergius (1884-1949), p. 62 in 'Chemie in unserer Zeit', VCH-Verlagsgesellschaft mbH, 19. Jahrgang, April 1985, Weinheim, Germany
- ^ Speight, James G. (2008). Synthetic Fuels Handbook: Properties, Process, and Performance. McGraw-Hill Professional. pp. 9–10. ISBN 9780071490238. http://books.google.com/?id=E3pgqnGgHjIC&pg=PA9. Retrieved 2009-06-03.
- ^ Lowe, Phillip A.; Schroeder, Wilburn C.; Liccardi, Anthony L. (1976). Technical Economies, Synfuels and Coal Energy Symposium, Solid-Phase Catalytic Coal Liquefaction Process. American Society of Mechanical Engineers. p. 35.
- ^ Höök, M., Aleklett, K., 2009. A review on coal to liquid fuels and its coal consumption, International Journal of Energy Research, article in press Link
- ^ Tarka, Thomas J.; Wimer, John G.; Balash, Peter C.; Skone, Timothy J.; Kern, Kenneth C.; Vargas, Maria C.; Morreale, Bryan D.; White III, Charles W.; Gray, David (2009). Affordable Low Carbon Diesel from Domestic Coal and Biomass. United States Department of Energy, National Energy Technology Laboratory. p. 21.
- ^ "Shenhua Group Starts China's First Coal-to-Fuel Plant". http://www.bloomberg.com/apps/news?pid=20601072&sid=a3SIXbVZ8JiE&refer=energy. Retrieved 11 August 2009.
- ^ Rao, P. N. (2007). "Moulding materials". Manufacturing technology: foundry, forming and welding (2 ed.). New Delhi: Tata McGraw-Hill. p. 107. ISBN 9780074631805.
- ^ Kirk, Edward (1899). "Cupola management". Cupola Furnace - A Practical Treatise on the Construction and Management of Foundry Cupolas. Philadelphia, PA: Baird. p. 95. OCLC 2884198.
- ^ "Kentucky: Secretary of State - State Mineral". 20 October 2009. http://sos.ky.gov/kids/all/symbols/mineral.htm. Retrieved 7 August 2011.
- ^ "Utah State Rock - Coal". Pioneer: Utah's Online Library. Utah State Library Division. http://pioneer.utah.gov/research/utah_symbols/rock.html. Retrieved 7 August 2011.
- ^ "NYMEX.com: Coal". http://www.nymex.com/coa_fut_descri.aspx. Retrieved 16 January 2008.
- ^ "ICE: Coal Futures". https://www.theice.com/coal.jhtml. Retrieved 16 January 2008.
- ^ Coal News and Markets (Archive) Department of Energy -- see Bloomberg for realtime prices
- ^ Toxic Air: The Case for Cleaning Up Coal-fired Power Plants Report by the American Lung Association http://www.lungusa.org/assets/documents/healthy-air/toxic-air-report.pdf
- ^ Environmental impacts of coal power: air pollution Union of Concerned Scientists http://www.ucsusa.org/clean_energy/coalvswind/c02c.html
- ^ MSNBC Staff and Service. (2004)"Deadly power plants? Study Fuels Debate: Thousands of Early Deaths Tied To Emissions." Retrieved 5 November 2008
- ^ World Coal Association "Environmental impact of Coal Use"
- ^ http://www.columbia.edu/~jeh1/2007/IowaCoal_20071105.pdf
- ^ Peckham, Jack (2002). "Diesel Fuel News: Ultra-clean fuels from coal liquefaction: China about to launch big projects - Brief Article". Diesel Fuel News. http://www.findarticles.com/p/articles/mi_m0CYH/is_15_6/ai_89924477. Retrieved 9 September 2005.
- ^ Si Tingting and Li Jing (2008-12-12). "Coal-to-liquids project rescheduled to launch in early 2009". China Daily. http://www.chinadaily.com.cn/bizchina/2008-12/12/content_7300666.htm. Retrieved 2008-12-12.
- ^ "Sasol, Shenhua Group May Complete Coal-to-Fuel Plant by 2013". http://www.bloomberg.com/apps/news?pid=newsarchive&sid=a.MSdPvb0Ep8. Retrieved 10 January 2009.
- ^ U.S. Energy Information Administration. 2008. World Coal Production, Most Recent Estimates 1980-2007 (October 2008). http://www.eia.doe.gov/emeu/international/coalproduction.html [accessed 11-2-08].
- ^ Swartz, Spencer; Oster, Shai (July 19, 2010). "China Becomes World's Biggest Energy Consumer". Wall Street Journal. http://online.wsj.com/article/SB10001424052748703720504575376712353150310.html?mod=djemalertNEWS. Retrieved 2010-07-19.
- ^ Feller, Gordon. "China’s Coal". ECOworld. http://www.ecoworld.com/home/articles2.cfm?tid=450. Retrieved 2010-07-19.
- ^ "Where The Coal Is Stained With Blood". TIME. March 2, 2007
- ^ Fisher, Juliya (2003). "Energy Density of Coal". The Physics Factbook. http://hypertextbook.com/facts/2003/JuliyaFisher.shtml. Retrieved 25 August 2006.
- ^ "How much coal is required to run a 100-watt light bulb 24 hours a day for a year?". Howstuffworks. http://science.howstuffworks.com/question481.htm. Retrieved 25 August 2006.
- ^ http://www.euractiv.com/energy/analysis-efficiency-coal-fired-power-stations-evolution-prospects/article-154672
- ^ CO2 Carbon Dioxide Emissions from the Generation of Electric Power in the United States, DOE, EPA, 1999
- ^ "Sino German Coal fire project". http://www.coalfire.caf.dlr.de/projectareas/world_wide_distribution_en.html. Retrieved 9 September 2005.
- ^ "Committee on Resources-Index". Archived from the original on 25 August 2005. http://web.archive.org/web/20050825231038/http://resourcescommittee.house.gov/archives/108/testimony/johnmasterson.htm. Retrieved 9 September 2005.
- ^ "http://www.fire.blm.gov/textdocuments/6-27-03.pdf" (PDF). http://www.fire.blm.gov/textdocuments/6-27-03.pdf. Retrieved 9 September 2005.
- ^ "EHP 110-5, 2002: Forum". http://ehp.niehs.nih.gov/docs/2002/110-5/forum.html. Retrieved 9 September 2005.
- ^ "Overview about ITC's activities in China". Archived from the original on 16 June 2005. http://web.archive.org/web/20050616004903/http://www.itc.nl/personal/coalfire/activities/overview.html. Retrieved 9 September 2005.
- ^ "Fire in The Hole". http://www.smithsonianmag.com/travel/firehole.html. Retrieved 5 June 2011.
- ^ "North Dakota's Clinker". http://www.state.nd.us/ndgs/ndnotes/ndn13_h.htm. Retrieved 9 September 2005.
- ^ "BLM-Environmental Education- The High Plains". Archived from the original on 12 March 2005. http://web.archive.org/web/20050312133748/http://www.blm.gov/education/high_plains/article.html. Retrieved 9 September 2005.
- ^ "http://www.wsgs.uwyo.edu/Coal/CR01-1.pdf" (PDF). Archived from the original on 12 September 2005. http://web.archive.org/web/20050912063721/http://www.wsgs.uwyo.edu/Coal/CR01-1.pdf. Retrieved 9 September 2005.
- ^ "EIA International Energy Statistics : Coal : Consumption". http://tonto.eia.doe.gov/cfapps/ipdbproject/IEDIndex3.cfm?tid=1&pid=1&aid=2. Retrieved 10 June 2009.
- ^ Coal: Resources and Future Production, 47 page report by Energy Watch group, March 28, 2007 (revised July 10, 2007)
- ^ World Energy Council - Survey of Energy Resources 2010
- ^ Alan Sherwood and Jock Phillips. 'Coal and coal mining - Coal resources', Te Ara - the Encyclopedia of New Zealand, updated 2-Mar-09 URL: http://www.TeAra.govt.nz/en/coal-and-coal-mining/2
- ^ "BP Statistical review of world energy 2011" (XLS). British Petroleum. June 2011. http://www.bp.com/liveassets/bp_internet/globalbp/globalbp_uk_english/reports_and_publications/statistical_energy_review_2011/STAGING/local_assets/spreadsheets/statistical_review_of_world_energy_full_report_2011.xls. Retrieved 2011-06-10.
- ^ World Steam Coal Flows.
- ^ World Coal Flows by Importing and Exporting Regions
- ^ EIA International Energy Annual
- ^ International Energy Annual
Further reading
- Walter Licht, Thomas Dublin (2005). The Face of Decline: The Pennsylvania Anthracite Region in the Twentieth Century. Cornell University Press. ISBN 0-8014-8473-1. OCLC 60558740.
- Long, Priscilla (1991). Where the Sun Never Shines: A History of America's Bloody Coal Industry. New York, NY: Paragon House. ISBN 1557784655. OCLC 25236866.
- Rottenberg, Dan (2003). In the Kingdom of Coal; An American Family and the Rock That Changed the World. Routledge. ISBN 0-415-93522-9. OCLC 52348860.
- Robert H. Williams and Eric D. Larson (December 2003). "A comparison of direct and indirect liquefaction technologies for making fluid fuels from coal" (PDF). Energy for Sustainable Development VII: 103–129. http://www.ieiglobal.org/ESDVol7No4/dclversussicl.pdf.
- Outwater, Alice (1996). Water: A Natural History. New York, NY: Basic Books. ISBN 0-465-03780-1. OCLC 37785911.
- Smith, Duane A. (May 1993). Mining America: The Industry and the Environment, 1800-1980. Lawrence, KS: University Press of Kansas. p. 210. ISBN 0870813064.
- Freese, Barbara (2003). Coal: A Human History. Penguin Books. ISBN 0-7382-0400-5. OCLC 51449422.
External links
- Energy KIDS – Coal page from USA's Department of Energy.
- European Association for Coal and Lignite
- Coal Online – International Energy Agency
- World Coal Association
|
||||||||||||||
|
|||||||||||||||||||||||||